ACTO recognized as a Major Contender in Everest Group’s PEAK Matrix®
Get the report
Field Force Effectiveness for Life Sciences
Ensure rep competency, skill, and confidence with ACTO
The preferred platform
Adopted by more pharma reps than any other learning experience platform, ACTO is the preferred solution in the industry.
Learn more
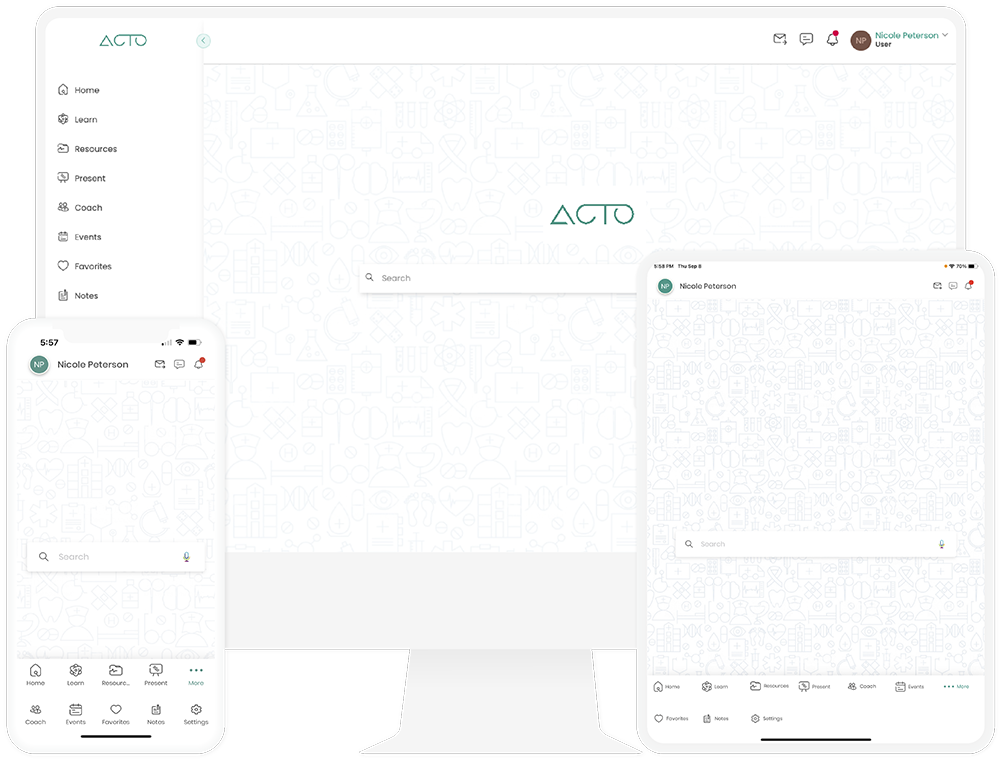
Our Platform
Why ACTO?
ACTO helps Life Sciences organizations educate and engage learners through unified touchpoints and journeys to reduce risk, improve efficiency, and boost effectiveness.
1
Reduce risk
Provide field reps with easy access to high-quality, MLR/PRC-approved training and content right at the point of need.
Read the report
2
Improve efficiency
Replace independent point solutions with a unified platform to consolidate and simplify your learner’s environment, while saving company resources.
Watch the video
3
Boost effectiveness
Surround field reps with what they need, when they need it, and how they want it, so they can sell in the most efficient and effective way possible.
Learn more
It's time to change
The “old way” of learning no longer works
0%
Knowledge retention one week after training
0%
Content never used by sales reps
0%
Sales reps can’t find the information they need
OUR SOLUTIONS
Made for Life Sciences
The ACTO platform was built specifically to meet the needs of the Life Sciences industry.
Commercial Learning & Development
Make training a joy for your reps and equip them for continuous learning in the flow of work.
Explore
Commercial Excellence
Directly impact new product, new indication, and new rep results by correlating learning to sales performance.
Explore
Medical Affairs
Ensure pull-through and maximum retention of clinical knowledge with your MSLs.
Explore
Information Technology
Save time, money, and resources by consolidating and simplifying your learning tech stack.
Explore
Our customers agree with us:

“ACTO has changed the way our sales force operates in a very positive way. We love how it speeds up our sales cycle, puts time back in our day and encourages continuous learning.”






